Scientists at the Indian Institute of Technology Kharagpur (IIT-KGP) have developed what they are calling a “highly accurate, portable, yet user-friendly, affordable and non-invasive device for detecting oral cancer in resource-constrained clinical settings.” (Image above of Professor Suman Chakraborty and his student Arka Bhowmik on the left and their affordable, non-invasive device on the right)
Giving credibility to their innovation, experts from the Guru Nanak Institute of Dental Sciences and Research in Kolkata in their “high standard biopsy reports” have established the efficacy of this method of differentiating cancerous and precancerous stages of “suspected oral abnormalities” through supervised clinical trials. With the first phase of clinical trials over, the next phase will see field deployments in the coming months in Odisha.
Under the guidance of Professor Suman Chakraborty, a professor in the Department of Mechanical Engineering the research work has recently been published in the prestigious Proceedings of the National Academy of Sciences, USA and is also selected for an eminent print journal called ‘In This Issue’.
Talking about the speed of diagnosis and affordability of this device, Prof Chakraborty tells The Better India, “Diagnosis is arrived at within 5 to 10 minutes of taking the image. The estimated cost per device is USD 500 (approx. Rs 37,000). The OCT (optical coherence tomography) machine costs at least USD 35,000 (approx. Rs 26 lakh), whereas CT Scan, MRI, etc. are significantly more expensive. More than the one time cost of such complex machines is the extreme high-end lab infrastructure along with supporting resources needed for their operation and maintenance that are by no means available in resource-limited settings.”
Once companies can manufacture this device at scale, the cost is expected to go down further.
How Does This Device Work?
The central innovation in this work can detect cancer of superficial locations in the body with very little margin of misclassification.
“This platform technology includes a portable handheld unit that combines various sensors and controllers and feeds the measured data to a computer simulation engine, which has been demonstrated for its high efficacy in clinical trials to classify normal, pre-cancer and cancer cases in the oral cavity, without needing referral to specialised medical centres for resource-intensive diagnostic procedures,” explains Prof Chakraborty.
The technology overcomes the compelling challenges of cancer screening in resource-limited settings where other forms of complementary diagnostics are not commonly available.
“This has been realised by offering a technology disruption via developing a simple yet automated touch-free approach of estimating blood flow variations in different regions of the potentially diseased tissue, specifically correlating with the diseased condition. This has proven to be technologically superior as compared to thermal imaging-based screening technologies currently in use since the temperature in the tissue itself varies with the surrounding conditions. Add combined variabilities in the local blood flow and metabolism to the mix, and you do not always have a specific indicator of the diseased state under investigation,” he explains.
Going further, scientists have also infused a machine learning-based classification approach with physics-based analytics based on thermal images obtained from portable devices.
“Since oral cancer, at its early stage, is known to manifest an increase in blood flow whereas full-grown cancer reveals a decrease in blood flow similar to pre-cancer or normal cases, such data-science augmented interpretation algorithm has minimised inevitable instances of misclassification due to similar common deceptive features among other medical conditions. This may be compounded by obvious inter-patient variability that, in borderline cases, may lead to wrong clinical decisions. The new technology is set to resolve such inevitable clinical dilemmas or potential misclassification by delivering a judicious amalgamation of data analytics and physics-based approach towards disease modelling and detection technology,” he says.
Components of This Device
The device is a portable and user-friendly blood perfusion imager (BPI) that combines a miniature far-infrared (FIR) camera and a humidity sensor, which are electronically controlled and interfaced with a combined physics-based and data-driven software engine. As explained further, the task of this device, which can be deployed in resource-limited settings, is classifying cancerous and different non-cancerous traits in an identified sub-surface tissue in-vivo.
“The physical device consists of a probing unit for screening, and a processing unit for obtaining blood perfusion data and disease recognition. The probing unit is composed of a holder and sensor housing, such that the holder is used for guiding the sensor housing to the measurement site and the sensor housing is used to maintain a stable environment for sensors to minimise the effect of breathing. The sensor housing consists of an on-chip long-wave infrared (IR) camera for measuring tissue temperature and a fully-calibrated digital humidity sensor for measuring ambient temperature and relative humidity inside the mouth. The IR camera sensor array captures the spectral radiance in the wavelength range of 8 – 14 µm (micrometre) and uses additional signal-processing electronics to convert the radiometric values into temperature value with thermal sensitivity < 50 mK (milli Kelvin) and for imaging at a rate of 8.7 Hz,” he says.
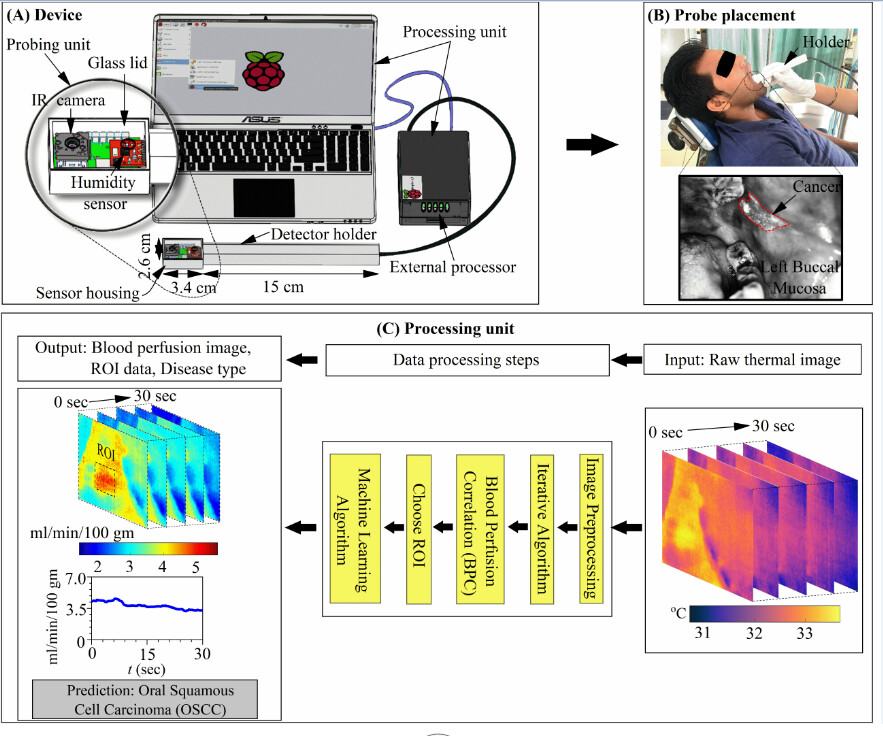
Why This Device Works in Resource-Constrained Settings
In resource-limited settings, there is a serious dearth of accurate yet affordable diagnostic tools to arrive at a decisive recommendation during the first possible clinical examination of the patient, possibly when the disease may otherwise be only in a pre-cancer or early cancer stage.
This challenge stems from various elusively overlapping features of oral cancer (i.e., oral squamous cell carcinoma) with certain common precancerous conditions (oral sub-mucosal fibrosis, oral leukoplakia, oral erythroplakia, and oral lichen planus) and several other types of mouth sores and ulcers. Without sophisticated resources, the clinician is compelled to rely more on sensibly recognisable features during the clinical examination of the at-risk oral sites (buccal mucosa, tongue, gum, palate, etc.), in addition to the patient history.
Often, their most practical resort is the golden subjective RULE (red and/or white lesion, ulcerated, lump, exceeding three weeks), presenting more dilemmas or ambiguities rather than providing definitive clinical decisions making guidelines in clinically confusing scenarios. Also, there are obvious barriers against accessing resource-intensive or expensive diagnostic facilities available only at super-speciality medical centres at prohibitively high costs.
“The new technology offers an invaluable possibility of detecting the potential vulnerable cases early enough, during a first examination by a clinician in a community health centre, saving millions of lives in the process. More vulnerable cases identified during the screening may promptly be referred to advanced speciality centres. As an inexpensive co-option to the standard and established clinical practices, this value-added tool is thus likely to strengthen the confidence of doctors in preliminary decision making. The technology is currently available for ready licensing to companies for commercial adaptation. Subsequently, it may be subjected to more extensive statutory field trials before clinical use. This may take about two years from now if companies take over the technology promptly,” claims Prof Chakraborty.
Motivation Behind Innovation
Work on this device started about four years ago. Spearheaded by Prof Chakraborty’s student and post-doctoral fellow Arka Bhowmik, they received support from Prof. Jyotirmoy Chatterjee, Head, School of Medical Science and Technology, and his student Biswajoy Ghosh. Prof Chatterjee and Ghosh helped provide OCT images for complementary interpretation. After the development of the device, it was validated by doctors from the Guru Nanak Institute of Dental Science and Research, who are acknowledged as co-authors in the paper published.
“Cancer of the oral cavity remains a major cause of morbidity and mortality in underserved communities, which reveals on-an-average 80% chance of five-year survival rate [meaning what per cent of people live at least 5 years after the cancer is found] if diagnosed early, whereas the survival rate drops to 65% or less in more advanced stages. In reality, most oral cancers remain undetected until reaching an advanced stage. In resource-limited settings, there is a serious dearth of accurate yet affordable diagnostic tools to arrive at a decisive recommendation during the first possible clinical examination of the patient, possibly when the disease may otherwise be only in a pre-cancer or early cancer stage,” he says.
The ongoing pandemic has exposed the challenges due to the non-availability of diagnostic technologies that are accurate yet low cost, accessible, user-friendly and amenable to massive manufacturing scale-up. The availability of such easy-to-use and reasonably sensitive detection methods for community-level testing holds the potential of capturing the commonly missed cases of early stages of the disease before it proceeds to a more advanced and virtually irrecoverable state. Such compelling needs, along with other prevailing public health challenges such as poor oral hygiene due to different forms of addiction inherent to the lifestyles of socially and economically challenged populations, have triggered an intense urge of developing this technology that essentially offers an amalgamated approach with a trade-off between the scientific standards of high-end laboratory-based tests with the elegance of common rapid tests.
“This paradigm appears to be the future of diagnostics, targeting specific public health measures towards catering the underserved, with no distinction between consumers having expected variabilities in economic barriers, and in the process ‘democratise’ disease diagnostics with no differential treatment of the rich and the poor,” concludes Prof Chakraborty.
(Edited by Yoshita Rao)
No comments:
Post a Comment